our products
Phoenix Pharmaceutical, Inc.
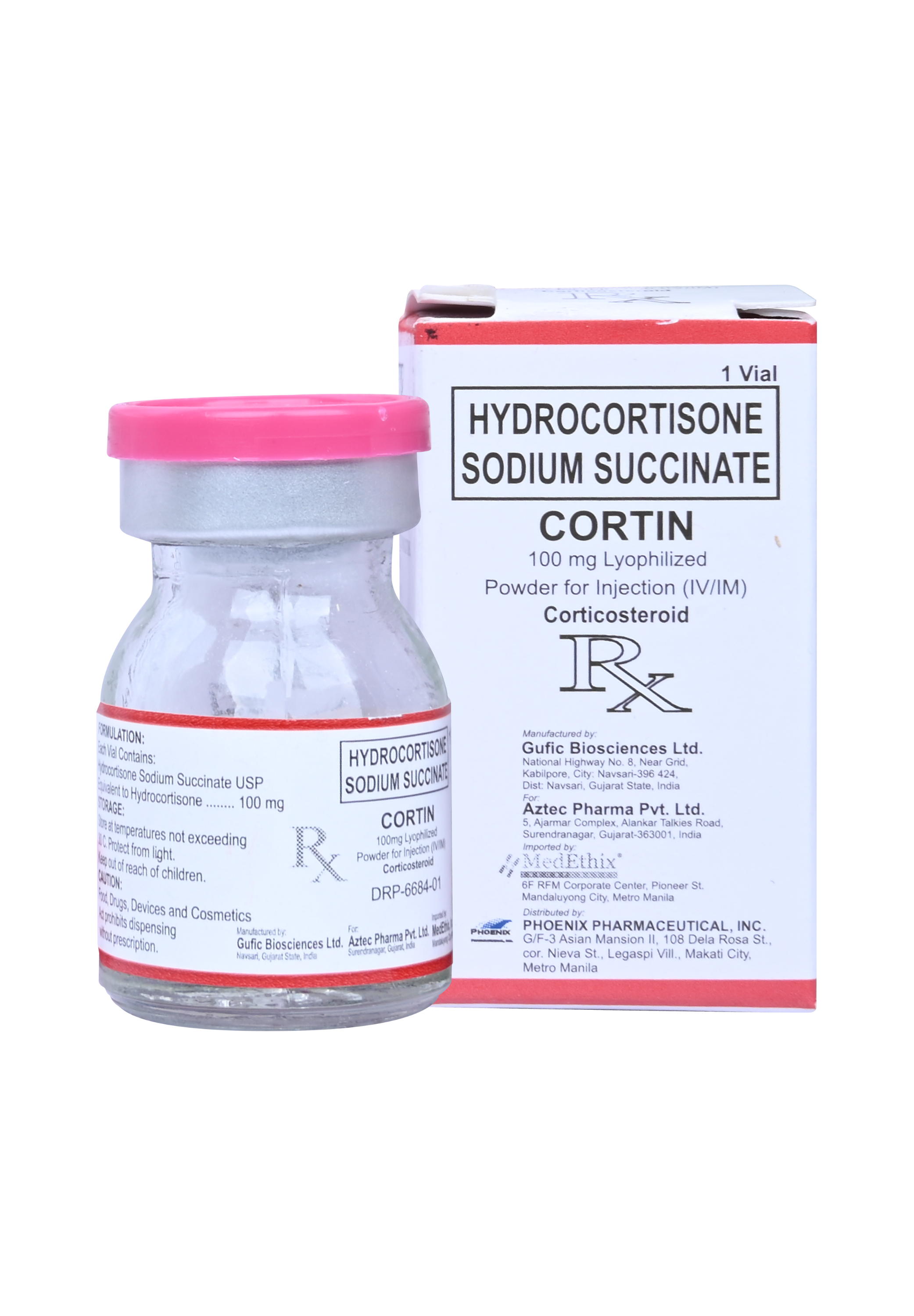
Hydrocortisone Sodium Succinate (CORTIN )
100 mg Lyophilized Powder for Injection (IM/IV)
This medication act as an anti-inflammatory agent and is part of the adrenal steroid class.
Being a short-acting glucocorticoid, effects typically last for about 8 to 12 hours, making it useful in managing conditions requiring short-term relief of inflammation or treatment of adrenal insufficiency.
Lower dose provides anti-inflammatory effects while higher doses are immunosuppressive.
COMPOSITION:
Each vial contains:
Hydrocortisone Sodium Succinate USP Equivalent to Hydrocortisone……………100 mg
ACTION:
CORTIN sterile powder is an anti-inflammatory glucocorticoid.
Naturally occurring glucocorticoids (hydrocortisone and cortisone), which also have salt-retaining properties, are used as replacement therapy in adrenocortical deficiency states. Their synthetic analogs are primarily used for their anti-inflammatory effects in disorders of many organ systems. Glucocorticoids cause profound and varied metabolic effects. In addition, they modify the body’s immune response to diverse stimuli.
INDICATION:
Use of CORTIN is indicated as follows: allergic states, dermatologic diseases, endocrine disorders, gastrointestinal diseases, hematologic disorders, neoplastic diseases, nervous system disorders, ophthalmic diseases, renal diseases, respiratory diseases, rheumatic disorders.
DOSAGE AND ADMINISTRATION:
Initial Dose: 100 mg to 500 mg, depending on the specific disease entity being treated. This dose may be repeated at intervals of 2, 4, or 6 hours as indicated by the patient’s response and clinical condition.
Inspect visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Use solution only if it is clear. Unused solution should be discarded after 3 days.
This preparation may be administered by intravenous injection, by intravenous infusion, or by intramuscular injection, the preferred method for initial emergency use being intravenous injection.
CONTRAINDICATION:
CORTIN sterile powder is contraindicated in systemic fungal infections and patients with known hypersensitivity to the product and its constituents.
Intramuscular corticosteroid preparations are contraindicated for idiopathic thrombocytopenic purpura.
WARNINGS/PRECAUTIONS:
Patients who are on corticosteroids are more susceptible to infections than healthy individuals. Administration of live or live, attenuated vaccines is contraindicated in patients receiving immunosuppressive doses of corticosteroids.
Use with caution in patients with congestive heart failure, hypertension, or renal insufficiency, as sodium retention with resultant edema and potassium loss may occur in patients receiving corticosteroids.
Persons who are on corticosteroids should be warned to avoid exposure to chicken pox or measles. If exposed, medical advice should be sought without delay.
STORAGE CONDITION:
Store at temperatures not exceeding 30˚C. Protect from light. Keep out of reach of children.
PRESENTATION:
USP Type I Clear Glass Vial (Box of 1’s)
The information on this website is intended for educational and informational purpose only. It is not a substitute for professional medical advice, diagnosis, or treatment. Always consult with a qualified healthcare provider before making any healthcare decisions or changes to your treatment plan.
Epinephrine (ADLINE)
1mg/mL Solution for Injection (IM/SC/IV)
Also known as Adrenaline.
Epinephrine is used chiefly as a stimulant in cardiac arrest, vasoconstrictor in shock, bronchodilator and antispasmodic in bronchial asthma.
COMPOSITION:
Each mL contains: Epinephrine………………………….. 1mg
DESCRIPTION:
Epinephrine (Adline) is a clear solution, sterile, colorless packed in 1 mL amber ampoule with white color break.
INDICATIONS:
Used in the emergency treatment of anaphylaxis and acute allergic reactions.
DOSAGE AND ADMINISTRATION:
The intramuscular (IM) route is recommended as the most appropriate for most individuals who have to give Epinephrine to treat an anaphylactic reaction. The patient should be monitored as soon as possible (pulse, blood pressure, ECG, pulse oximetry).
The best site for IM Injection is the anterolateral aspect of the middle third of the thigh.
Do not inject into the buttocks, digits, hands or feet.
The subcutaneous route for Epinephrine is not recommended for treatment of an anaphylactic reaction as it is less effective.
Adults:
The usual dose is 0.5 mg (0.5mL of Epinephrine 1 mg/mL (1:1000). If necessary, this dose may be repeated several times at 5-minute intervals according to blood pressure, pulse and respiratory function.
Paediatric population:
The following doses of Epinephrine 1 mg/mL (1:1000) Solution for Injection are recommended:
Age:
Over 12 years
Dose:
0.5 mg IM (0.5 mL 1mg/mL (1:1000) solution)
0.3 mg IM (0.3 mL 1mg/mL (1:1000) solution) if the child is small or
pre-pubertal)
6 – 12 years
Dose:
0.3 mg IM (0.3 mL 1mg/mL (1:1000) solution)
6 months – 6 years
Dose:
0.15 mg IM (0.15 mL 1mg/mL (1:1000) solution)
Under 6 months
Dose:
0.01 mg/kg IM (0.01 mL/kg 1mg/mL (1:1000) solution)
If necessary these doses may be repeated several times at 5-15 minutes intervals according to blood pressure, pulse and respiratory function.
CONTRAINDICATIONS:
Hypersensitivity to the active substance or to any of the excipients.
Contraindications are relative as this product is intended for use in life-threatening emergencies.
WARNING AND PRECAUTIONS:
This product is for emergency use only and medical supervision of the patients is necessary after administration.
Epinephrine 1 mg/mL (1:1000) Solution for Injection is not suitable for IV use.
PREGNANCY AND LACTATION:
Epinephrine should only be used during pregnancy if the potential benefits outweigh the possible risks to the fetus. Epinephrine is distributed into breast milk, breast-feeding should be avoided in mothers receiving Epinephrine injection
STORAGE CONDITIONS:
Store at temperatures not exceeding 25°C. Protect from moisture and light.
DOSAGE FORM AND PACKAGING AVAILABLE:
Dosage form: Solution for Injection
Packaging available: USP Type I amber glass ampoule x 1 mL (Box of 10’s).
The information on this website is intended for educational and informational purpose only. It is not a substitute for professional medical advice, diagnosis, or treatment. Always consult with a qualified healthcare provider before making any healthcare decisions or changes to your treatment plan.


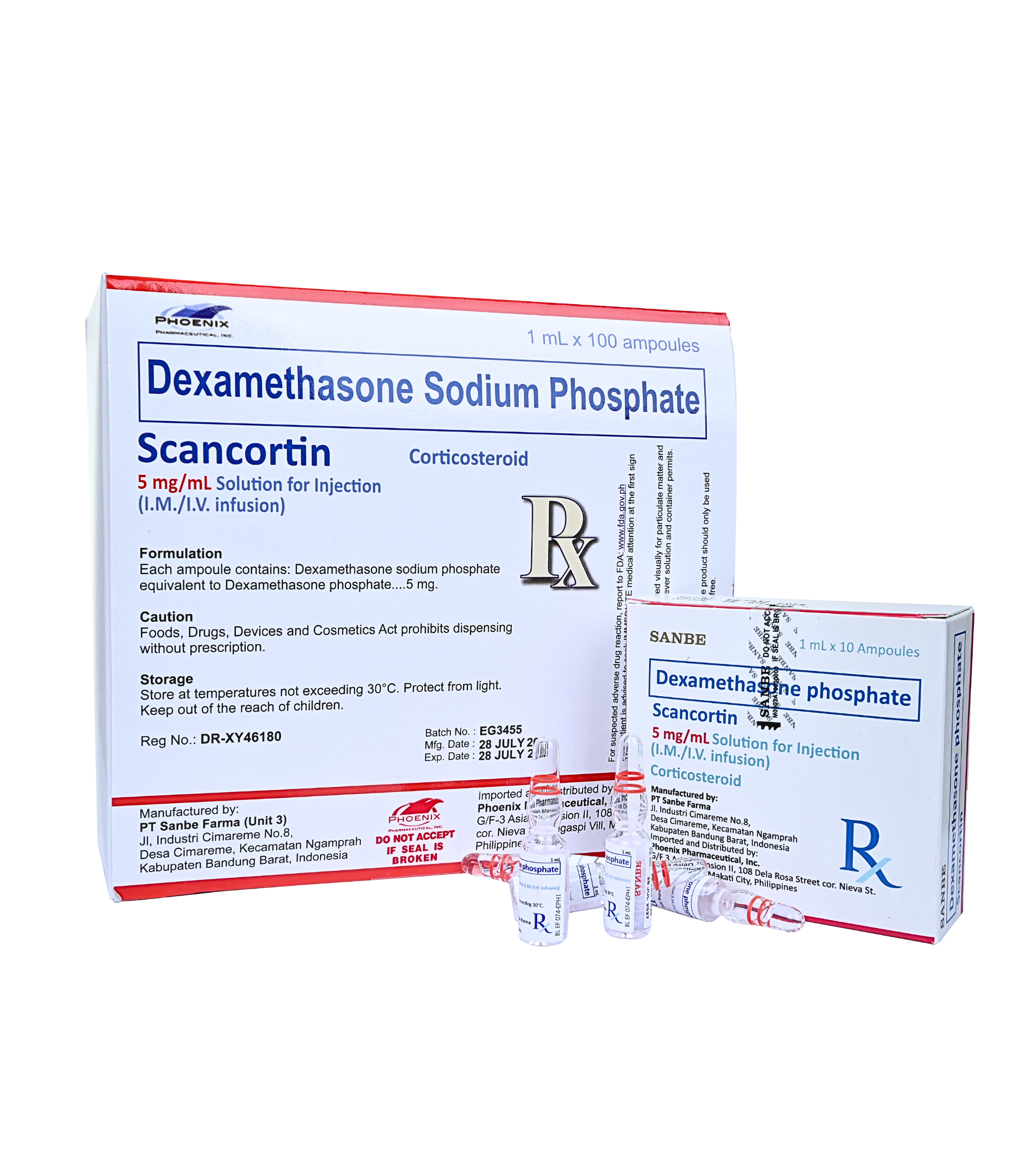
Dexamethasone Sodium Phosphate (SCANCORTIN )
5 mg/mL Solution for Injection (I.V./I.M.)
This drug is highly potent and the most powerful long lasting systemic corticosteroid available in the market. The potency is 6-10 times than that of prednisone,
prednisolone and approximately 25 times more potent than the short acting product hydrocortisone. It has a duration of action ranging from 24-72 hours, with a rapid onset of action, 5 minutes after administration.
Scancortin is widely used for its powerful anti-inflammatory properties, particularly in treating severe allergic reaction, asthma and autoimmune conditions.
Each ampoule contains:
Dexamethasone sodium phosphate equivalent to Dexamethasone phosphate 5mg
DESCRIPTION:
Scancortin contains in aqueous solution dexamethasone sodium phosphate. Dexamethasone is currently the most powerful glucocorticosteroid available in the market. Its potency is 6-10 times that of prednisone and prednisolone and 25 times that of hydrocortisone. Despite its powerful effect, however, dexamethasone has been proven to possess minimal salt and water retention properties.
ACTION:
Being a powerful glucocorticosteroid, Scancortin has strong anti-inflammatory, anti-toxic, anti-shock, anti-allergic, anti-pyretic and immunosuppressive properties. Also, the aqueous solution of Scancortin permits a rapid diffusion of the solution into the body fluids thus producing a quick penetrating effect.
INDICATIONS:
Due to its powerful and fast action, peak blood levels are reached within 5 minutes after injection, thus making it an ideal preparation for life-threatening conditions and other emergency cases.
Scancortin is highly recommended for anaphylactic shock, severe allergic reactions, fulminating infections, cerebral edema. Scancortin can also be used intrathecally in painful joints and acute rheumatic disorders. It is also indicated as a starter dose or when oral therapy is impossible or impractical.
DOSAGE:
Scancortin can be given by intravenous, intramascular, sub-cutaneous, intrathecal or by I.V. drip. Normally, one injection of 5 mg is sufficient when given daily.
However, in more serious cases, 2-3 injections may be required depending on the severity of the condition and the response of the patient. In severe cases of shock such as in toxemia or serious forms of allergies, the dosage may be increased proportionately to counteract the severe stress present.
PRECAUTION AND SIDE-EFFECTS:
Like any other glucocorticosteroid, Scancortin should also be used with caution in patients with tuberculosis, mental disorders, hypertension, cardiomyopathy, renal insufficiency, and other conditions contra-indicated to corticosteroid therapy. In cases of emergencies where the life of the patient is in danger, however, large doses may be given for short periods of time despite the presence of contraindications.
PRESENTATION:
Ampoules of 1 mL containing 5 mg. Box of 10×10’s
The information on this website is intended for educational and informational purpose only. It is not a substitute for professional medical advice, diagnosis, or treatment. Always consult with a qualified healthcare provider before making any healthcare decisions or changes to your treatment plan.

Paracetamol (MAVEDOL)
150 mg/mL Solution for Injection (IM/IV)
Paracetamol is indicated for the short-term management of moderate pain, especially following surgery, and for the short-term treatment of fever.
Intravenous administration is recommended when there is an urgent need to manage pain or reduce fever, and when other routes of administration, such as oral or rectal, are not feasible.
FORMULATION:
Each ml contains
Paracetamol……150mg
DESCRIPTION:
Mavedol is a clinically proven analgesic and antipyretic.
ACTION:
Mavedol produces analgesia by raising the threshold of the pain center in the brain and by obstructing
impulse at the pain mediating chemoceptors. The drug produces antipyresis by an action on the hypothalamus, heat dissipation is increased as a result of vasodilation and increased peripheral blood flow.
PHARMACOKINETICS:
Mavedol distribution to most body tissues and fluids is both rapid and uniform. Time to peak plasma
concentration: approximately 15 minutes. The plasma half-life is 1.25 to 3 hours but may be increased by liver damage and following overdose. It crosses the placenta and is present in breast milk. Metabolized predominantly in the liver and excreted in the urine.
INDICATION:
Pyrexia of unknown origin, fever and pain, associated with common childhood disorders, tonsillitis, upper respiratory tract infection, post-immunization reactions, post operative fever, after tonsillectomy and other conditions, where patient is unable to take oral medications but where Paracetamol can be administered with advantage for prevention of febrile convulsion, headache, cold, sinusitis, muscle pain, arthritis and toothaches.
CONTRAINDICATION:
Hypersensitivity to Paracetamol. Repeated administration is contraindicated in patients with anemia, cardiac, pulmonary, renal, and hepatic disease.
DOSAGE AND ADMINISTRATION:
Intramuscular route:
Adults: 2-3 ml every 4 to 6 hours.
Children: (2-12 years/ > 33kg): Up to 2 ml every 4 to 6 hours. Below 2 years of age: Half to 1 ml every 4 to 6 hours. Intravenous route: Slow I.V Administration.
CAUTION:
Foods, Drugs, Devices, and Cosmetics Act prohibit dispensing without prescription.
STORAGE CONDITION:
Store at temperatures not exceeding 30°C. Protect from light.
PRESENTATION: Ampoule of 150mg/ml (300mg/2ml)
The information on this website is intended for educational and informational purpose only. It is not a substitute for professional medical advice, diagnosis, or treatment. Always consult with a qualified healthcare provider before making any healthcare decisions or changes to your treatment plan.


Prednisolone (Predisyr)
15mg/ 5mL Syrup 30 mL
It is a systemic synthetic corticosteroid that exerts a wide range of physiologic effects similar to naturally occurring glucocorticoids.
It used to treat variety of inflammatory diseases including severe asthma, rheumatoid arthritis,
allergic reactions, severe skin conditions, and some blood disorders.
Predisyr syrup is formulated with a pleasant strawberry flavor making it more palatable and suitable for children.
COMPOSITION:
15 mg of Prednisolone in each 5 ml
DESCRIPTION:
Prednisolone Syrup (Predisyr) contains prednisolone which is a glucocorticoid. Glucocorticoids are adrenocortical steroids both naturally occurring and synthetic. Prednisolone is a man-made form of a natural substance (corticosteroid hormone) made by the adrenal gland.
ACTION:
Predisyr is a corticosteroid with mainly glucocorticoid activity. Glucocorticoid actions are wide-ranging.
They have potent anti-inflammatory and immunosuppressive effects. They also have profound and varied metabolic effects.
INDICATION:
Predisyr is highly recommended in all conditions responsive to glucocorticoid therapy, such as
respiratory diseases, dermatologic diseases, endocrine disorders, collagen diseases, allergic states, ophthalmic diseases, hematologic disorders, neoplastic diseases, edematous states, as well as adjunctive short term therapy for rheumatic disorders.
DOSAGE AND ADMINISTRATION: Dosage of Predisyr syrup should be individualized according to the severity of the disease and the response of the patient.
For infants and children the recommended dosage should be governed by the same considerations rather than strict adherence to the ratio indicated by age or body weight. The initial dosage in adults may vary from 5 to 60 mg per day depending on the specific disease entity being treated.
Take with food to avoid stomach irritation.
CONTRAINDICATIONS: Systemic fungal infections
CAUTION:
Foods, Drugs, Devices, and Cosmetics Act prohibit dispensing without prescription.
STORAGE CONDTION:
Keep this medicine out of the sight and reach of children. Store at temperature not exceeding 30˚C.
PRESENTATION: Predisyr Syrup: 30 ml amber bottle
The information on this website is intended for educational and informational purpose only. It is not a substitute for professional medical advice, diagnosis, or treatment. Always consult with a qualified healthcare provider before making any healthcare decisions or changes to your treatment plan.

Cotrimazine (TRIZINE )
205 mg/ 45 mg per 5 mL Suspension
A drug consisting of sulfadiazine 205 mg and trimethoprim 45mg when combined in vitro, the components show high activity and a high frequency of synergy against travelers’ diarrhea, respiratory and urinary tract infections.
Cotrimazine suspension is rapidly and almost completely absorbed from the gastrointestinal tract. Peak blood concentration is reached within 1 to 4 hours.
This rapid absorption profile ensures effective blood concentrations are quickly reached, allowing the drug to act efficiently against the targeted infections.
This suspension is formulated with a pleasant orange flavor making it more palatable and suitable for children.
FORMULATION:
Each teaspoonful (5mL) contains:
Sulfadiazine………………………….. 205 mg
Trimethoprim………………………….45 mg
DESCRIPTION:
Cotrimazine (Trizine) is a broad-spectrum antibacterial combination of sulfadiazine and trimethoprim with powerful bactericidal effects. The ideal combination of the two antibacterial components is based on the results of scientific research whereby the combination exerts a sequential blockade on two consecutive steps in the biosynthesis of nucleic acids and proteins essential to many pathogenic bacteria. The blockade results in the two components potentiating each other’s action that broadens the antibacterial spectrum and reduces the risk of the emergence of resistant strains.
Although individually, sulfadiazine and trimethoprim are bacteriostatic in nature, the combination produces a profound bactericidal action. Sulfadiazine is particularly suitable to combine with trimethoprim because of its favorable pharmacokinetic properties.
INDICATIONS:
Infections cause by gram-positive and gram-negative bacteria sensitive to sulfadiazine and trimethoprim such as respiratory and urinary tract infections. Cotrimazine’s (Trizine) broad antibacterial spectrum includes Streptococci, Staphylococci, Pneumococci, Haemophilus influenzae, Escherichia coli, Klebsiella spp and the majority of Proteus spp.
DOSAGE:
Recommended dose
Suspension
CONTRAINDICATIONS:
Hypersensitivity to sulfonamides and trimethoprim, severe liver damage, blood dyscracias e.g. megaloblastic anemia and severe renal insufficiency. Cotrimazine (Trizine) should not be given to premature and newborn babies during their first 2 months of life.
PRECAUTION:
Patient allergic to sulfonamides or trimethoprim may be allergic to sulfadiazine and trimethoprim combinations.
Patients allergic to furosemide, thiazide diuretics, sulfonylureas, or carbonic anhydrase inhibitors may be allergic to sulfonamides also.
SPECIAL PRECAUTIONS:
G6PD deficiency, AIDS; actual or possible folate deficiency; perform regular hematological examination; child with fragile X chromosome associated with mental retardation; discontinue in case of skin rash.
PREGNANCY AND LACTATION:
FDA Pregnancy Category C.
STORAGE:
Store at temperatures not exceeding 30°C
AVAILABILITY:
Suspension: 60 mL amber bottle
The information on this website is intended for educational and informational purpose only. It is not a substitute for professional medical advice, diagnosis, or treatment. Always consult with a qualified healthcare provider before making any healthcare decisions or changes to your treatment plan.


Cotrimazine (TRIZINE FORTE)
820 mg/ 180 mg Tablet
A drug consisting of sulfadiazine 820 mg and trimethoprim 180mg when combined in vitro, the components show high activity and a high frequency of synergy against a variety of infections of the respiratory tract, urinary tract and gastrointestinal tract.
Antibacterial Formulation:
Each Trizine Forte® tablet contains:
Sulfadiazine…………………………………….820mg
Trimethoprim………………………………….180mg
DESCRIPTION:
Trizine Forte® is a broad spectrum antibacterial combination of sulfadiazine and trimethoprim with powerful bactericidal effects. The ideal combination of the two antibacterial components is based on the results of scientific research whereby the combination exerts a sequential blockade on two consecutive steps in the biosynthesis of nucleic acids and proteins essential to many pathogenic bacteria. The blockade results in the two components potentiating each other’s action that broadens the antibacterial spectrum and reduces the risk of emergence of resistant strains.
Although individually, sulfadiazine and trimethoprim are bacteriostatic in nature, the combination produces a profound bactericidal action. Sulfadiazine is particularly suitable to combine with trimethoprim because of its favorable pharmacokinetic properties.
INDICATION:
Infections caused by gram-positive and gram-negative bacteria sensitive to sulfadiazine and trimethoprim such as in respiratory and urinary tract infections. Cotrimazine’s broad antibacterial spectrum includes Streptococci, Staphylococci, Pneumococci, Haemophilus influenzae, Escherichia coli, Klebsiella spp and the majority of Proteus spp.
CONTRAINDICATION:
Hypersensitivity to sulfonamide and trimethoprim, severe liver damage, blood dyscrasias e.g. megaloblastic anemia and severe renal insufficiency.
SPECIAL PRECAUTION:
G6PD deficiency, AIDS, actual or possible folate deficiency; perform regular hematological examination; child with fragile X chromosome associated with mental retardation; discontinue in case of skin rash.
PRECAUTION:
Patients allergic to furosemide, thiazide diuretics, sulfonylureas, or carbonic anhydrase Inhibitors may be allergic to sulfonamide also. Patient allergic to sulfonamide or trimethoprim may be allergic to sulfadiazine and trimethoprim combinations.
DOSAGE:
Recommended dose: One tablet two times a day.
STORAGE CONDITION:
Store at room temperature NOT exceeding 30° C, away from heat, light, and moisture.
The information on this website is intended for educational and informational purpose only. It is not a substitute for professional medical advice, diagnosis, or treatment. Always consult with a qualified healthcare provider before making any healthcare decisions or changes to your treatment plan.

It would increase urination to help drain excess fluid (edema) due to heart failure, liver cirrhosis, or kidney disease.
It is also indicated in urgent medical crises that require rapid loss of water like fluid buildup in the lungs (pulmonary edema) or fluid overload due to heart failure.
Furosemide (FUROSCAN)
20 mg/2mL Solution for Injection (IM/IV)
COMPOSITION:
Each ampoule (2mL) contains: Furosemide……………………………………20mg
PRODUCT DESCRIPTION:
Furosemide (Furoscan) is a potent ‘loop’ diuretic with rapid action. It exerts an inhibiting effect on electrolyte reabsorption not only in the proximal and distal tubules of the kidney but also in the ascending loop of Henle. After intravenous injection, its effects are evident within 5 minutes and lasts for about 2 hours.
PHARMACODYNAMICS AND PHARMACOKINETICS:
Furosemide (Furoscan) is administered parenterally. Furosemide (Furoscan) duration of action is relatively brief (2 to 4 hours). Furosemide (Furoscan) is secreted into the urine.
INDICATION:
Due to its strong and rapid effects, Furosemide (Furoscan) is highly recommended for acute cases where a rapid action is needed. Furosemide (Furoscan) is also indicated in all forms of cardiac edema that cannot be corrected by sufficient glycoside therapy. Furosemide (Furoscan) is further indicated in cardiac insufficiency, lung edema, ascites in cirrhosis of the liver, threatening anuria, failing kidney function, edema due to burns and mild to moderate hypertension.
INTERACTIONS:
Aminoglycosides antibiotics may potentiate ototoxicity when administered with any loop diuretic; Nonsteroidal Anti- Inflammatory Drugs (NSAIDS) may hamper the diuretic response to furosemide; same as Ethacrynic acid may potentiate the effects of Warfarin.
DOSAGE AND MODE/ROUTE OF ADMINISTRATION:
Injection: Usual adult dose is 20-40 mg (1-2 amps). Higher doses may be required in cases of acute or chronic renal failure (250-500 mg in 225-450 mL sodium chloride solution infused over 1-2 hours). This has to be determined by the attending doctor. For children, a dose of 10 mg is sufficient.
CAUTION:
Foods, Drugs, Devices, and Cosmetics Act prohibit dispensing without prescription.
STORAGE CONDITION:
Store at temperatures not exceeding 30°C.
AVAILABILITY:
Ampoule of 2 mL containing 20 mg, boxes of 100’s
The information on this website is intended for educational and informational purpose only. It is not a substitute for professional medical advice, diagnosis, or treatment. Always consult with a qualified healthcare provider before making any healthcare decisions or changes to your treatment plan.


Oxytocin (OBCIN ) 10 I.U.
5mL Solution for Injection (I.M./I.V. Infusion)
Oxytocin is used to induce labor or strengthen uterine contractions, or to control bleeding after childbirth.
Oxytocin is also used to stimulate uterine contractions in a woman with an incomplete or threatened miscarriage.
COMPOSITION:
Each ml contains:
Oxytocin (synthetic) BP……………………………………………..10 I.U.
DESCRIPTION:
Oxytocin is an essential hormone for childbirth and lactation. The hormone is produced in the hypothalamus and secreted by the posterior pituitary.
Obcin Injection contains synthetic oxytocin which is completely free from traces of vasopressin or extraneous animal protein. As a result, Obcin reduces the risk of elevated blood pressure and minimize the risk of allergic and hypersensitivity reactions.
ACTIONS:
The pharmacological and clinical actions of Obcin Injection are identical with those of natural purified oxytocin. It has a direct stimulatory effect on the smooth muscle of the uterus that initiates and increases uterine contractions without blood pressure elevation. The effectivity of Obcin Injection progressively increases at term due to the increased uterine sensitivity to oxytocic activity.
INDICATION:
Induction of labour, inadequate uterine effort and post-partum hemorrhage.
DOSAGE:
Induction of labour:
Obcin should be administered as an intravenous infusion or preferably, by means of a variable-speed infusion pump. The initial infusion rate should be set at 1-4 mU/min (2-8 drops/min). It may be gradually increased at intervals not shorter than 20 min.
Caesarean section:
5 IU by slow intravenous injection immediately after delivery.
Prevention of postpartum uterine haemorrhage:
5 IU slowly intravenous or 5-10 IU IM after delivery of the placenta. For induction or enhancement of labour, the infusion should be continued at an increased rate during the third stage of labour and for the next few hours thereafter.
Treatment of postpartum uterine haemorrhage:
5 IU slowly I.V. or 5-10 IU I.M., followed in severe cases by I.V. infusion 5-20 IU of Oxytocin in 500 ml of a non-hydrating diluent, run at the rate necessary to control uterine atony.
Incomplete, inevitable, or missed abortion:
5 IU slowly I.V. or 5-10 IU I.M. if necessary followed by I.V. infusion at a rate of 20-40 mU/min or higher. CONTRAINDICATION:
Obstacles to delivery, e.g, abnormal position of the child and cephalo-pelvic disproportion.
STORAGE CONDITION:
Store at temperatures 2°C-8°C.
(Under refrigeration. No freezing)
KEEP OUT OF REACH OF CHILDREN
The information on this website is intended for educational and informational purpose only. It is not a substitute for professional medical advice, diagnosis, or treatment. Always consult with a qualified healthcare provider before making any healthcare decisions or changes to your treatment plan.

Paracetamol (ADAMOL)
150 mg/mL Solution for Injection (IM/IV)
Paracetamol is indicated for the short-term management of moderate pain, especially following surgery, and for the short-term treatment of fever. Intravenous administration is recommended when there is an urgent need to manage pain or reduce fever, and when other routes of administration, such as oral or rectal, are not feasible.
The information on this website is intended for educational and informational purpose only. It is not a substitute for professional medical advice, diagnosis, or treatment. Always consult with a qualified healthcare provider before making any healthcare decisions or changes to your treatment plan.
Epinephrine (ACCEPHRINE)
1mg/mL Solution for Injection (IM/SC/IV)
Also known as Adrenaline.
Epinephrine is used chiefly as a stimulant in cardiac arrest, vasoconstrictor in shock, bronchodilator and antispasmodic in bronchial asthma.
The information on this website is intended for educational and informational purpose only. It is not a substitute for professional medical advice, diagnosis, or treatment. Always consult with a qualified healthcare provider before making any healthcare decisions or changes to your treatment plan.

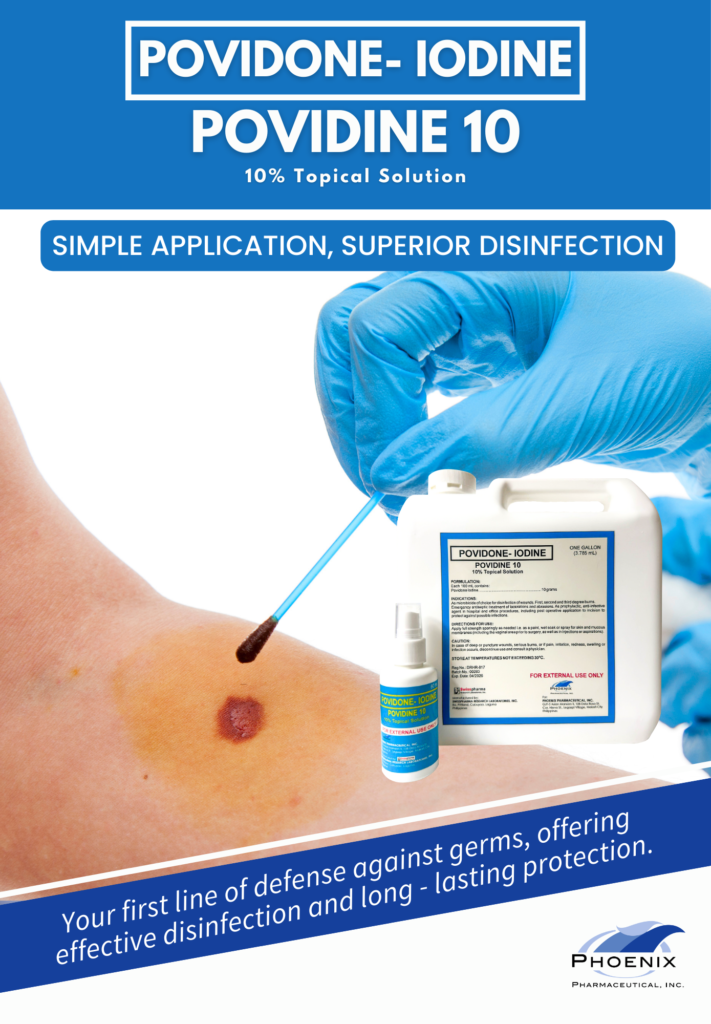
POVIDONE - IODINE (POVIDINE 10)
10% Topical Solution
---
FORMULATION:
Each 100 mL contains: Povidone Iodine………………………………………………………………………………………….10 grams
INDICATION:
As microbicide of choice for disinfection of wounds. First, second and third degree burns.
Emergency antiseptic treatment of lacerations and abrasions. As prophylactic, anti-infective agent in hospital and office procedures,
including post operative application to incision to protect against possible infections.
DIRECTIONS FOR USE:
Apply full strength sparingly as needed i.e. as a paint, wet soak or spray for skin and mucous membranes (including the vaginal
area prior to surgery, as well as in injections or aspirations).
CAUTION:
In case of deep or puncture wounds, serious burns, or if pain, irritation, redness, swelling or infection occurs, discontinue use and
consult a physician.
STORE AT TEMPERATURES NOT EXCEEDING 30° C.
Reg No.: DRHR-817
FOR EXTERNAL USE ONLY
The information on this website is intended for educational and informational purpose only. It is not a substitute for professional medical advice, diagnosis, or treatment. Always consult with a qualified healthcare provider before making any healthcare decisions or changes to your treatment plan.

POVIDONE - IODINE (POVIDINE 7.5%)
7.5% Surgical Scrub
DIRECTIONS FOR USE:
A. FOR PRE-OPERATIVE WASHING BY OPERATING PERSONNEL Wet hands with water. Pour about 5 mL (one teaspoonful) of Povidine 7.5% on palms and spread over both hands. Without adding more water, rub Povidine 7.5% thoroughly over all areas including fingernails for about five minutes, use brush if desired. Add a little water to develop copious suds and thoroughly rinse under running water. Complete the wash by scrubbing with another 5 mL Povidine 7.5% in the same way.
B. FOR PRE-OPERATIVE USE ON PATIENT After the skin area is shaved, wet with water. Apply Povidine 7.5% (1 mL is sufficient to cover an area of 20-30 square inches) and rub thoroughly for about five minutes to develop a lather and rinse off by aid of sterile gauze saturated with water. The area may then be painted with Povidine 10% Antiseptic Solution and allowed to dry.
C. FOR USE IN THE PHYSICIAN’S OFFICE Use for washing whenever a germicidal soap is indicated, for maximum degerming of the hands proceed as under “A”. Moreover, to prepare the patients skin, proceeds as under “B”.
STORE AT TEMPERATURES NOT EXCEEDING 30° C.
Reg No.: DRHR-817
FOR EXTERNAL USE ONLY ONE GALLON (3.785 mL)
The information on this website is intended for educational and informational purpose only. It is not a substitute for professional medical advice, diagnosis, or treatment. Always consult with a qualified healthcare provider before making any healthcare decisions or changes to your treatment plan.


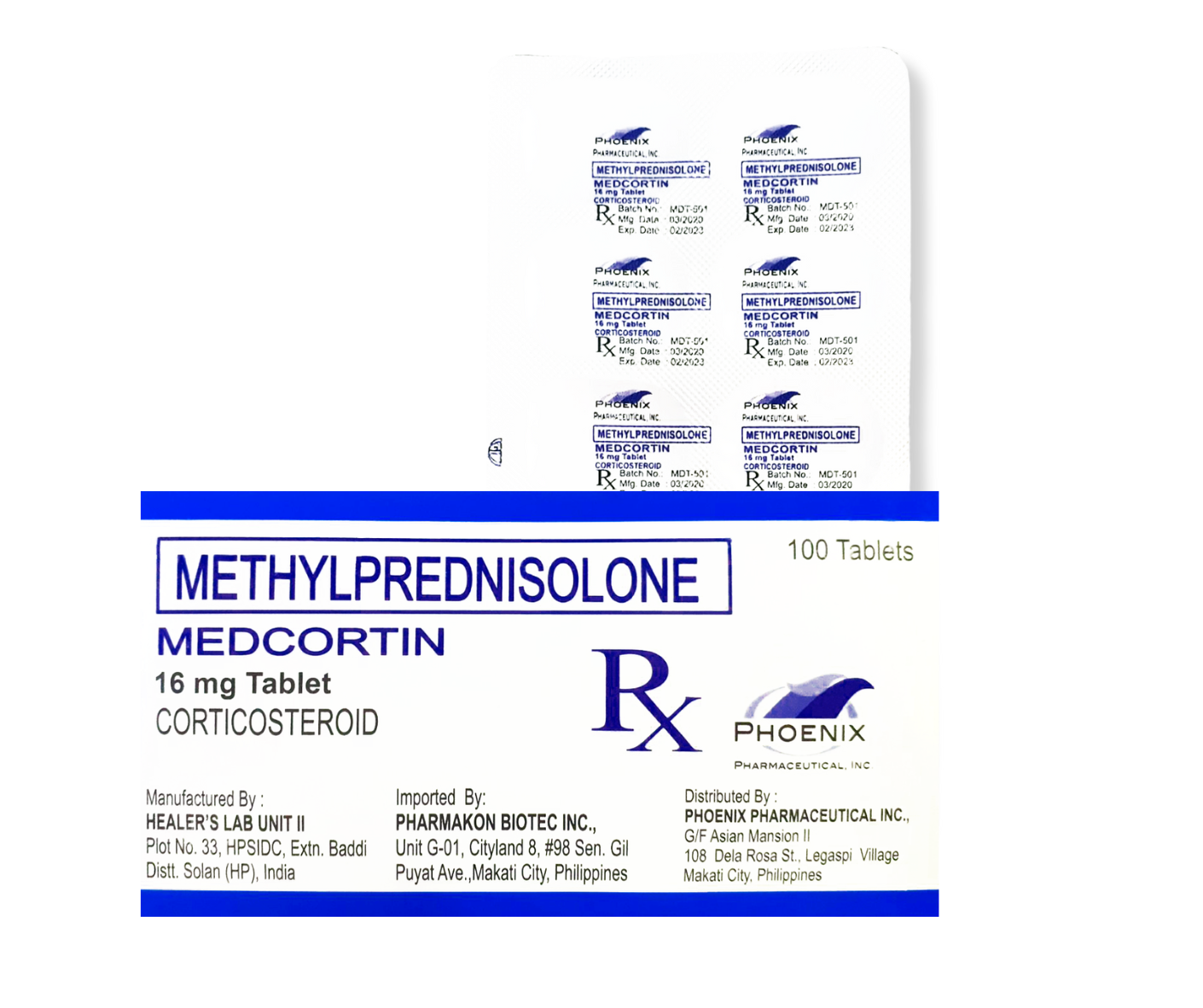
Methylprednisolone (MEDCORTIN)
16mg Tablet
It is a systemic synthetic corticosteroid that exerts a wide range of physiologic effects similar to naturally occurring glucocorticoids.
.
The information on this website is intended for educational and informational purpose only. It is not a substitute for professional medical advice, diagnosis, or treatment. Always consult with a qualified healthcare provider before making any healthcare decisions or changes to your treatment plan.